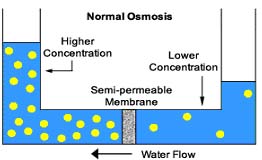
Osmosis is a physical force. It is the natural tendency of water with a low concentration of dissolved particles to move across a semi-permeable membrane to an area of water with a high concentration of dissolved particles. The water will try to reach an equilibrium on both sides. I.e. both sides of the semi-permeable membrane will have the same concentration of dissolved particles. This is how plants absorb nutrients from the soil. Picture a tea bag placed in a mug of hot water. (The tea bag is the semi-permeable membrane). At first, the water is free of tea. However, with time, the tea will appear to seep from the tea bag into the mug. This is the process of osmosis. If you were to leave the tea bag in the mug for long enough, the concentration of tea inside the teabag would equal the concentration of tea |
 |
| |
|
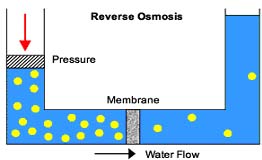 |
Now for reverse osmosis The process of reverse osmosis requires that the water be forced through a semi-permeable membrane (the tea bag from the previous example) in the opposite direction of the natural osmotic flow; leaving the dissolved particles in the more highly concentrated solution.
In order for reverse osmosis to occur, the amount of force or pressure applied must exceed the osmotic pressure. |
|
A semi-permeable membrane is at the heart of a reverse osmosis system Reverse osmosis works through a technique called membrane separation. The membrane is permeable only to water molecules.
|
| |
|
 |
Two types of Membranes:
CTA membrane - cellulose triacetate
TFC membrane - thin film composite |
|
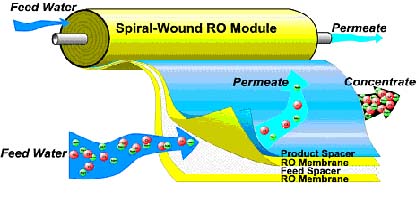
Raw water enters a module housing the membrane system. The water is forced against the semi-permeable membrane and only clean water molecules pass through the pores in the membrane. Impurities are rejected and flushed away. |
| |
|
| Crossflow Filtration |
|
| |
|
While the principles of reverse osmosis are simple the process can not run indefinitely unless steps are taken to ensure the membrane does not become clogged by impurities |
| |
|
To significantly reduce the rate of membrane fouling, reverse osmosis systems employ crossflow filtration. In conventional filtration, the entire water solution to be filtered is pumped through the filter media and all contaminants too large to pass through the pores of the membrane are trapped or retained on the surface. |
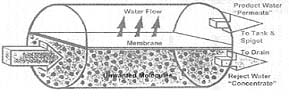 |
In crossflow filtration, two exit streams are generated -- a "concentrate" stream (reject water) containing those material which are rejected or do no pass through the membrane, and the "permeate" stream (product water) which has been pumped through the membrane, and passes to the tank |
| |
|
| The comparative size of particles |
|
| |
|
Various mineral salts, heavy metals, particular matter, some organic molecules, bacteria and even viruses are rejected or repelled by the membrane surface based on their molecular or atomic weight. A second barrier, such as ultraviolet light, should be used if bacteria are present. The ability of the membrane to reject or repel dissolved particles, while allowing water to readily permeate, is based on the incredibly small size of the multitude of pores that penetrate its surface. Such pores are able to reject substances as small as 0.0005 microns. A micron (m) is a metric unit of length equal to a millionth of a meter, or 0.00003937 inch. A human hair is approximately 75 m in diameter. The smallest particle that can be seen with the naked eye is 40 m across The smallest bacteria is about 0.22 m while a virus is even smaller at 0.01 m. |
| |
|
Reverse Osmosis will remove the following contaminants: |
| |
|
Contaminant |
% nominal rejection |
Contaminant |
% nominal rejection |
Aluminum |
96-98 |
Ammonium |
80-90 |
Arsenic |
98-99% |
Borate |
30-50 |
Bacteria |
99+ |
Bromide |
90-95 |
Boron |
50-70 |
Calcium |
93-98 |
Cadmium |
93-97 |
Chromate |
85-95 |
Chloride |
92-95 |
Cyanide |
85-95 |
Copper |
96-98 |
Hardness Ca & Mg |
93-97 |
Fluoride |
92-95 |
Lead |
95-98 |
Iron |
96-98 |
Magnesium |
93-98 |
Manganese |
96-98 |
Nickel |
96-98 |
Mercury |
94-97 |
Orthophosphate |
96-98 |
Nitrate |
90-95 |
Polyphosphate |
96-98 |
Phosphate |
95-98 |
Radioactivity |
93-97 |
Potassium |
93-97 |
Silicate |
92-95 |
Silica |
80-90 |
Sodium |
92-98 |
Silver |
93-96 |
Thoisulfate |
96-98 |
Sulfate |
96-98 |
|
|
Zinc |
96-98 |
|
|
|
|
| |
|
Reverse osmosis is a separation process that uses pressure to force a solvent through a membrane that retains the solute on one side and allows the pure solvent to pass to the other side. More formally, it is the process of forcing a solvent from a region of high solute concentration through a membrane to a region of low solute concentration by applying a pressure in excess of the osmotic pressure. This is the reverse of the normal osmosis process, which is the natural movement of solvent from an area of low solute concentration, through a membrane, to an area of high solute concentration when no external pressure is applied. The membrane here is semipermeable, meaning it allows the passage of solvent but not of solute.
The membranes used for reverse osmosis have a dense barrier layer in the polymer matrix where most separation occurs. In most cases the membrane is designed to allow only water to pass through this dense layer while preventing the passage of solutes (such as salt ions). This process requires that a high pressure be exerted on the high concentration side of the membrane, usually 2-17 bar (30-250 psi) for fresh and brackish water, and 40-70 bar (600-1000 psi) for seawater, which has around 24 bar (350 psi) natural osmotic pressure which must be overcome. This process is best known for its use in desalination (removing the salt from sea water to get fresh water), but has also purified naturally occurring freshwater for medical, industrial process and rinsing applications since the early 1970s |
| |
|
| Method |
|
| |
|
When two solutions with different concentrations of a solute are mixed, the total amount of solutes in the two solutions will be equally distributed in the total amount of solvent from the two solutions. This is achieved by diffusion, in which solutes will move from areas of higher concentration to areas of lower concentrations until the concentration in all the different areas of the resulting mixture are the same, a state called equilibrium.
Instead of mixing the two solutions together, they can be put in two compartments where they are separated from each other by a semipermeable membrane. The semipermeable membrane does not allow the solutes to move from one compartment to the other, but allows the solvent to move. Since equilibrium cannot be achieved by the movement of solutes from the compartment with high solute concentration to the one with low solute concentration, it is instead achieved by the movement of the solvent from areas of low solute concentration to areas of high solute concentration. When the solvent moves away from low concentration areas, it causes these areas to become more concentrated. On the other side, when the solvent moves into areas of high concentration, solute concentration will decrease. This process is termed osmosis. The tendency for solvent to flow through the membrane can be expressed as "osmotic pressure", since it is analogous to flow caused by a pressure differential.
In reverse osmosis, in a similar setup as that in osmosis, pressure is applied to the compartment with high concentration. In this case, there are two forces influencing the movement of water: the pressure caused by the difference in solute concentration between the two compartments (the osmotic pressure) and the externally applied pressure. In the same way as in conventional osmosis, the solute cannot move from areas of high pressure to areas of low pressure because the membrane is not permeable to it. Only the solvent can pass through the membrane. When the effect of the externally applied pressure is greater than that of the concentration difference, net solvent movement will be from areas of high solute concentration to low solute concentration, and reverse osmosis occurs |
| |
|
|